FDA’s approval to a new arthritis drug
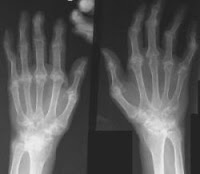 A new arthritis drug from Johnson & Johnson has been given a green signal by the Food and Drug Administration (FDA). The drug named Simponi (golimumab) has the potential to cure three forms of arthritis that occur when the immune system of the body attacks the joints causing pain and stiffness and making the movement difficult.
A new arthritis drug from Johnson & Johnson has been given a green signal by the Food and Drug Administration (FDA). The drug named Simponi (golimumab) has the potential to cure three forms of arthritis that occur when the immune system of the body attacks the joints causing pain and stiffness and making the movement difficult.The drug can be taken by those suffering moderate-to-severe rheumatoid arthritis and other similar chronic disorders of the joints including active ankylosing spondylitis (a chronic inflammatory arthritis of the spine) and active psoriatic arthritis (arthritis associated with psoriasis).
According to Dr. Bob Rappaport, M.D., director of the Division of Anesthesia, Analgesia, and Rheumatology Products in the FDA’s Center for Drug Evaluation and Research, “Today’s approval provides another treatment option for patients with these three debilitating disorders. And the steps we’re taking to minimize the risks will give patients the same level of safety protection required for other drugs in its class.”
To be injected once a month under the skin, Simponi should be taken along with methotrexate, an immunosuppressant drug, by those having rheumatoid arthritis. For patients with psoriatic arthritis, Simponi can either be used alone or in combination with methotrexate. However, for those suffering ankylosing spondylitis, Simponi is recommended to be used alone.
The drug blocks tumor necrosis factor (TNF), a form of protein that is likely to cause inflammation of tissue, cartilage and bones, when it is overproduced in the human body.
The drug is developed and marketed by Centocor Ortho Biotech Inc. based in Pennsylvania. However, outside the U.S., Simponi will be co-marketed by Schering-Plough Corp. which is based in Kenilworth, New Jersey.
President, Centocor Ortho Biotech, Kim Taylor stated, “With the approval of Simponi, we enhance our commitment to delivering effective and innovative treatments to the millions of patients living with chronic inflammatory diseases while expanding our immunology portfolio.
“Importantly, as patient safety remains our top priority, we have collaborated with the FDA to develop a Risk Evaluation and Mitigation Strategy to help ensure the risks of ant-TNF therapy are appropriately managed by doctors prescribing and patients receiving Simponi,” Taylor affirmed.
According to IMS Health Inc., a data research firm, Johnson & Johnson’s top-selling drug Remicade had brought in $ 5 billion of sales revenue last year.
Company’s new drug Simponi is likely to fetch annual revenue of more than $ 1 billion for Johnson & Johnson, revealed Jeff Jonas, an analyst working for Gabriel & Co. in Rye, New York. No wonder, Merck & Co., based in Whitehouse Station, New Jersey, is shelling out $41.1 billion to acquire Schering-Plough.
“It's a big drug,” revealed Jonas. “It'll definitely be over $1 billion, which is blockbuster status, but it'll probably reach $2 billion or $3 billion over the next few years,” he claimed.
The drug will face competition from Amgen Inc.’s Enbrel and Abbott Laboratories’ Humira.
The drug carries a warning which alerts health care professionals and patients about the risk of tuberculosis and serious fungal infections. Among the most common side effects of Simponi include nasal congestion, sore throat and upper respiratory tract infection.
In U.S. alone, there are nearly 3 million people who suffer from these chronic inflammatory diseases out of which nearly 2 million people have Rheumatoid arthritis.
Shares of J&J fell 48 cents to close at $50.92 Friday.
Source : www.themoneytimes.com














Post a Comment