New drug may help MS patients walk

A new drug may make getting around easier for the 2.5 million people worldwide afflicted with multiple sclerosis. It is the first medication to specifically target walking challenges faced by MS patients.
In January, the U.S. Food and Drug Administration approved Ampyra (dalfampridine) as an oral medication to improve walking in people with MS. It has been available by prescription since March 1.
The drug holds promise for Chris Messina, who misses strolling around the Staten Island Mall, and Danielle Mino, who can’t always keep up with her two young children.
Both Great Kills women have MS, a chronic disease that affects the central nervous system and can make walking a daunting experience.
Since April 21, Mrs. Messina has been taking one 10-milligram tablet of Ampyra twice a day. She’s been told it may take up to three months to experience results.
“I wait for a difference every day,” said Mrs. Messina, a 54-year-old former radiology clerk at Staten Island University Hospital.
Diagnosed in 1975, her ability to walk deteriorated about eight years ago during a relapse from which she never regained her former mobility.
“When I walk, I stumble, I trip, I walk crazy. I go back and forth and swagger,” said Mrs. Messina. “I used to live at the mall, but since I’ve been like this I haven’t been there in years.”
In two clinical trials of 540 MS patients in the United States and Canada, those treated with Ampyra had faster walking speeds on timed 25-foot walks than those given a placebo, according to Acorda Therapeutics, Inc., the Hawthorne, New York-based developer of the drug. Those whose walking speed improved, also noted an improvement in leg strength.
While the measuring stick for improvement may seem small, to those who live with MS, the trial’s findings are huge.
“My leg kind of drags a little, I don’t really have too much balance or strength especially in my right leg,” said Mrs. Mino, a 40-year-old mother of a 5½-year-old daughter and 4-year-old son.
“On a good day I can get up, get my children ready for school and then do what I have to do, cleaning, food shopping,” she explained. “But on a bad day, I just get my kids ready for school, put them on the school bus and then I just sit down most of the day.”
Ampyra is taken with other MS drugs and does not keep MS from getting worse. There is no cure for MS.
Both Mrs. Messina and Mrs. Mino are infused with Tysabri — a disease modifying medication that helps prevent relapses of MS symptoms — every four weeks at the MS Center of Staten Island in New Dorp.
“Ampyra is a symptomatic medicine. It makes you walk better, move better, but it doesn’t affect the disease itself,” said Dr. Allan Perel, director of the MS Center.
“You can still have attacks with it. You could still have progressive neurological findings. The MRIs could look worse.”
So far, more than 2,000 U.S. physicians have written at least one prescription for Ampyra, said Jeff Macdonald, a spokesman for Acorda. The MS Center has about 100 patients who have been prescribed the new drug, said Perel.
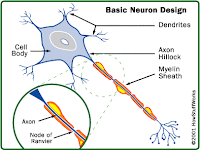
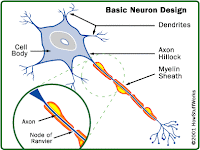
Ampyra is a potassium channel blocker which is believed to improve the transmission of nerve signals in nerve fibers whose insulating coating has been damaged by MS.
The tablets work as a timed-release formulation of dalfampridine, which was originally used as a seizure-inducing bird-control repellent.
For more than two decades, dalfampridine has been compounded in pharmacies and used off-label to treat people with various neurologic conditions.
While the FDA approved the use of Ampyra for people with any type of MS, it should not be taken by individuals with a history of seizures, or by those with moderate to severe kidney problems.
According to the FDA, when Ampyra is given at doses greater than 10 milligrams twice a day, it can cause seizures. The most common side effects reported by patients taking Ampyra in clinical trials included urinary tract infections, insomnia, dizziness, headache, nausea, back pain and balance problems.
The price of Ampyra is $1,056 for a 30-day supply (two tablets per day). It is only available by mail order through a network of specialty pharmacies that work with Acorda.
The drug’s hefty price is being offset by Acorda, which has developed assistance programs to help MS patients afford the drug.
Mrs. Mino is currently waiting for her prescription of Ampyra to arrive. Diagnosed with MS in 1999, she hopes the drug will make it easier to do activities like going on vacation with her children.
During a November trip to Disney World, trekking between attractions was especially challenging for her.
“It was so hard trying to walk through the park. At least every 10 minutes I would have to stop,” said Mrs. Mino. “Whenever we had to wait on a line for a ride, I was happy because I didn’t have to do any more walking.”
read more» Read more...












.jpg)