Metals could forge new cancer drug
 Drugs made using unusual metals could form an effective treatment against colon and ovarian cancer, including cancerous cells that have developed immunity to other drugs, according to research at the University of Warwick and the University of Leeds.
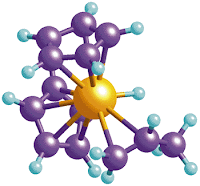
Drugs made using unusual metals could form an effective treatment against colon and ovarian cancer, including cancerous cells that have developed immunity to other drugs, according to research at the University of Warwick and the University of Leeds.The study, published in the Journal of Medicinal Chemistry, showed that a range of compounds containing the two transition metals Ruthenium and Osmium, which are found in the same part of the periodic table as precious metals like platinum and gold, cause significant cell death in ovarian and colon cancer cells.
The compounds were also effective against ovarian cancer cells which are resistant to the drug Cisplatin, the most successful transition metal drug, which contains the metal platinum.
Dr Patrick McGowan, one of the lead authors of the research from the School of Chemistry at the University of Leeds, explains: "Ruthenium and Osmium compounds are showing very high levels of activity against ovarian cancer, which is a significant step forward in the field of medicinal chemistry.
Sabine H. van Rijt, lead researcher in the laboratory of Professor Peter Sadler in the Department of Chemistry at the University of Warwick, said:
"Most interestingly, cancerous cells that have shown resistance to the most successful transition metal drug, Cisplatin, show a high death rate with these new compounds."
Professor Sadler, at the University of Warwick, commented that he is "excited by the novel design features in these compounds which might enable activity to be switched on and off".
Cisplatin was discovered in the 1970s and is one of the most effective cancer drugs on the market, with a 95% cure rate against testicular cancer. Since the success of Cisplatin, chemists all over the world have been trying to discover whether other transition metal compounds can be used to treat cancer.
In this type of anti cancer drug transition metal atoms bind to DNA molecules which trigger apoptosis, or programmed cell death, in the cancerous cells.
The study is a collaboration between the universities of Warwick and Leeds and was funded by the Engineering and Physical Sciences Research Council (EPSRC).
read more» Read more...